News Story
UMD ChBE Researchers Continue Search for Li-Battery Stabilization
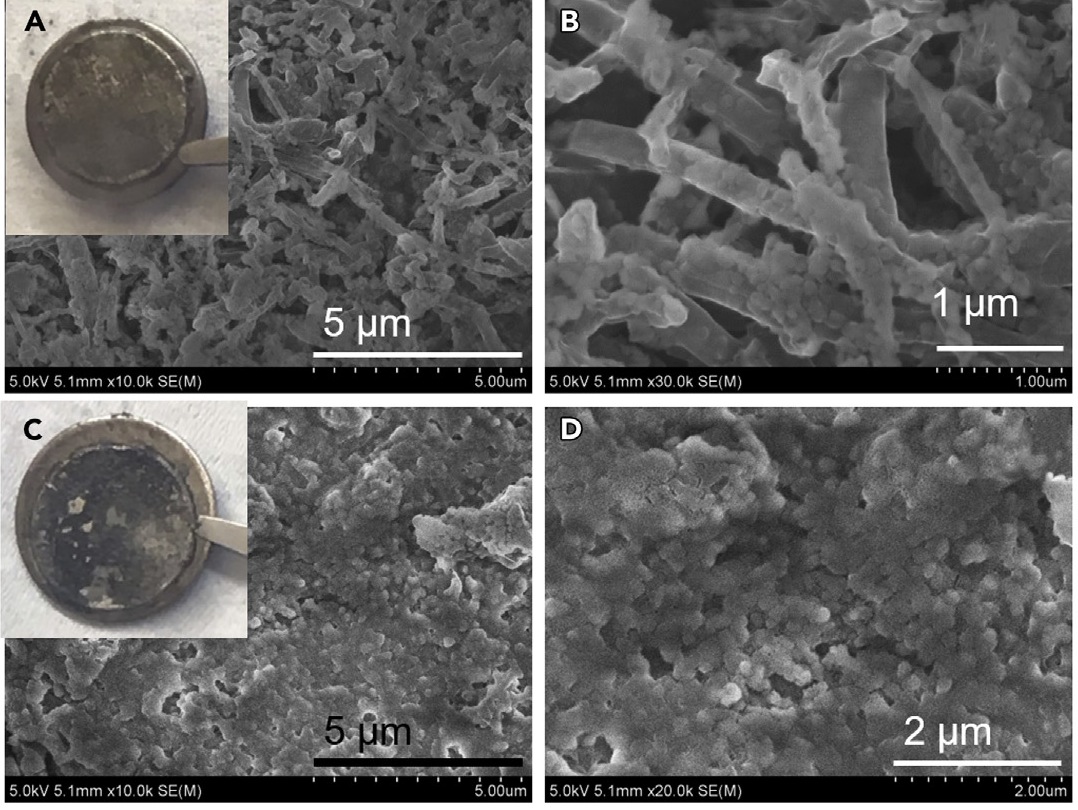
SEM images of cycled Li-metal anodes obtained from 1 M LiFSI EC/DMC (EC:DMC = 1:1) (A and B) and from 10 M LiFSI EC/DMC (C and D). Insets in (A) and (C) show the optical images of cycled Li foils on the spacers.
A healthy brain cell, or neuron, looks similar to a tree with a full canopy. The ‘branches’ are called dendrites, which receive electrical signals from other cells. Those signals are spread throughout the body and end in simple actions, such as wiggling a finger or blinking an eye; thus, they are absolutely necessary to healthy brain function.
In battery chemistry, however, dendrite growth is a real problem, often leading to significant performance reduction and raising safety concerns. Lithium (Li) metal – an ideal anode material due to its exceptional charge and storage capability – carries a high dendrite growth risk, which can lead to short-circuiting and spontaneous combustion. (Millimeter-sized ‘shadows’ cast by dendrites growing inside a Li-ion battery can be seen in real time and at high resolution by a new MRI method.)
Despite this issue, Li-metal is still regarded as the ‘holy grail’ in the scientific community, which is why researchers at the University of Maryland (UMD) continue their quest to solve the dendrite issue.
Researchers in the UMD Department of Chemical and Biomolecular Engineering (ChBE) - led by ChBE Professor Chunsheng Wang - have recently created a battery chemistry that successfully suppressed dendrite formation in Li-metal batteries by increasing the LiFSI (Lithium bis[fluorosulfonyl]imide) salt concentration in the electrolyte.
“Dendrite formation in the Li battery could penetrate the separator and cause serious safety issues,” said Xiulin Fan, ChBE research scientist and first author on the corresponding research paper. “This FSI anion in the concentrated electrolyte will react with the Lithium metal anode to generate a LiF-rich SEI layer, which can suppress dendrite formation and greatly improve the coulombic efficiency (describes the speed of energy transfer in electrochemical reactions) for the Li metal anode.”
Currently, in the commercial Li-ion battery, the anode is graphite. “The capacity of the graphite is only less than 372 mAh/g,” Dr. Fan continued. “The Li-metal anode can deliver a capacity of as high as 3860 mAh/g. So, if we replace the graphite with a Li-metal anode, the capacity and the energy density for the Li-battery could be doubled. This is especially important for the consumer electronics and electric vehicle market.”
For additional information:
Xiulin Fan, Long Chen, Xiao Ji, Tao Deng, Singyuk Hou, Ji Chen, Jing Zheng, Fei Wang, Jianjun Jiang, Kang Xu and Chunsheng Wang. “Highly Fluorinated Interphases Enable High-Voltage Li-Metal Batteries.” CHEM 4, 1-12; January 11, 2018. DOI: 10.1016/j.chempr.2017.10.017.
Published January 31, 2018